The diagnostic challenges presented by neurodegenerative conditions such as Alzheimer’s disease (AD) include issues of prevalence, under-diagnosis, and cost.
An ACCESS Biomarker Collection collaboration with Beckman Coulter provides you with a neurodegenerative disease research roadmap that centers on four key areas spanning our portfolio of diagnostic solutions:
The Role of APOE in Alzheimer's Disease:
Genetic Variants, Mechanisms, and Therapeutic Implications.
by Kinal Bhatt, MD, MPH
The current methods of testing to accurately detect elevated levels of amyloid plaque are inherently limited in their ability to scale.
Positron Emission Topography (PET) scanning
Cerebral Spinal Fluid (CSF) testing with mass spectrometry
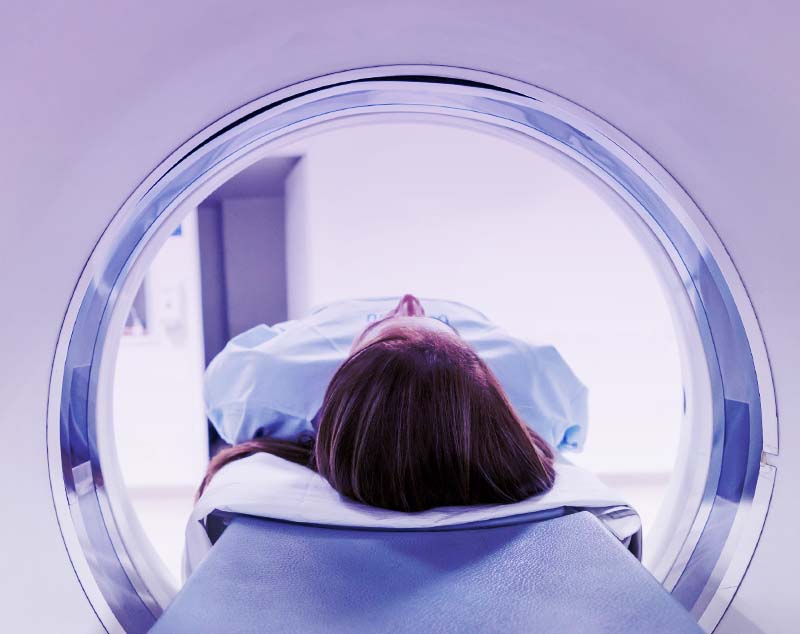

Blood-based biomarker testing offers the patient accessibility and cost efficiency benefits necessary to accelerate the move from diagnostic to therapeutic availability.
Blood-based biomarker immunoassay testing
Over the past 30 years, the pharma industry has invested more than $42.6 billion in Alzheimer’s disease therapeutic clinical trials.
At AAIC (Alzheimer’s Association International Conference) 2025, the Alzheimer’s Association unveiled Clinical Practice Guidelines (CPG) focused on the role of blood-based biomarker tests in assessing levels of Alzheimer’s disease pathology in people with cognitive impairment.
The following CPG recommendations apply to patients with cognitive impairment being seen in specialized care for memory disorders:

To learn more about the expanding role of blood-based biomarkers in Alzheimer’s disease research and how ACCESS to the NeuroABC Biomarker research use only collection positions your lab for the future, request a consultation with a Beckman Coulter Neurodegenerative disease biomarker specialist.
Learn more
Plasma p-Tau217 levels have shown correlation with amyloid pathology, which has implications for identifying neurodegenerative diseases such as Alzheimer’s disease. Measuring p-Tau217 may help to further research into AD pathology, flag samples from those with early-stage AD pathology, and help distinguish AD from other forms of dementia.
An ACCESS Biomarker Collection collaboration with Beckman Coulter is a giant step towards blood-based Alzheimer’s disease testing that:Blood-based Neurodegenerative Disease Assays (RUO)
| p-Tau217 | IFU | DOWNLOAD PDF |
| GFAP | IFU | DOWNLOAD PDF |
| NfL | IFU | DOWNLOAD PDF |
| APOE ε4 | IFU | DOWNLOAD PDF |
| β-Amyloid 1-42 | IFU | COMING SOON |
| BD-Tau | IFU | COMING SOON |
Research Use Only Assays in Development
| MTBR-Tau (CSF-based) |
| TDP-43 |
Assay has been granted Breakthrough Device Designation (BDD) from the U.S. FDA
| p-Tau217/ß-AmyIoid 1-42 Plasma Ratio* |
The population is aging and testing volume is poised to explode. In a field that’s advancing so rapidly, you need to be ready for what’s next. Now, there’s a way to position your lab for the future while supporting today’s core operations!